Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Micheau, C.; Ueda, Yuki; Motokawa, Ryuhei; Akutsu, Kazuhiro*; Yamada, Norifumi*; Yamada, Masako*; Moussaoui, S. A.*; Makombe, E.*; Meyer, D.*; Berthon, L.*; et al.
Journal of Molecular Liquids, 401, p.124372_1 - 124372_12, 2024/05
Supramolecular organization of extractant molecules impacts metal ions separation behavior. Probing bulk and interfacial structures of the relevant systems is expected to provide key insights into the metal ion selectivity and kinetic aspects. The supramolecular features of two solvent extraction systems based on malonamide extractants THMA in toluene and DBMA in n-heptane were studied using small-angle X-ray scattering for the organic bulk phases, as well as interfacial tension and neutron reflectivity measurements for the interfaces. In the bulk solution, THMA forms dimeric/trimeric associates but no aggregates in toluene, while DBMA forms large aggregates in n-heptane. On the other hand, THMA accumulates in a diffuse layer at the interface at high THMA concentration, whereas DBMA forms a compact but thinner layer. After Pd(II) extraction, the thickness of interfacial layers decreases in the case of THMA, and totally vanishes in the case of DBMA. Based on these new structural information, two mechanisms are proposed for Pd(II) and Nd(III) extraction with malonamides. In toluene, THMA associates slightly accumulate in the vicinity of the interface, then coordinate Pd(II) and diffuse into the organic bulk phase. In n-heptane, DBMA aggregates adsorb at the interface then pick up Nd(III) cations in their polar cores and finally diffuse into the bulk.
Sasaki, Yuji; Kaneko, Masashi; Ban, Yasutoshi; Suzuki, Hideya*
Journal of Nuclear Science and Technology, 11 Pages, 2023/11
Times Cited Count:0 Percentile:0.01(Nuclear Science & Technology)The mutual separation of Am and Cm is conducted using an alkyl-diamide amine (ADAAM) extractant. ADAAM exhibits extremely high separation factor with respect to Am and Cm separation (5.9) in a nitric acid- -dodecane system. The batch-wise multistage extractions are performed using a system containing 0.2 M ADAAM and 1.5 M nitric acid. In this multistage extraction, an organic solvent give 96.5% and 1.06% yields of Am and Cm. After the mutual separation of Am and Cm, an additional extraction step is included to reduce the volumes of these aqueous and organic phases. Taking these steps, Am and Cm can be recovered in just two or three stages in the aqueous phases.
-dodecane system. The batch-wise multistage extractions are performed using a system containing 0.2 M ADAAM and 1.5 M nitric acid. In this multistage extraction, an organic solvent give 96.5% and 1.06% yields of Am and Cm. After the mutual separation of Am and Cm, an additional extraction step is included to reduce the volumes of these aqueous and organic phases. Taking these steps, Am and Cm can be recovered in just two or three stages in the aqueous phases.
Sasaki, Yuji; Kaneko, Masashi; Ban, Yasutoshi; Kinoshita, Ryoma; Matsumiya, Masahiko*; Shinoku, Kota*; Shiroishi, Hidenobu*
Analytical Sciences, 39(9), p.1575 - 1583, 2023/09
Times Cited Count:0 Percentile:0(Chemistry, Analytical)Extraction of Rh from HCl can be performed by NTAamide(C6) (hexahexyl-nitrilotriacetamide) and other related compounds into n-dodecane. We use ion-pair extraction of anionic species of Rh-chloride and protonated extractant. Rh behave as anion in hydrochloric acid and the tertiary nitrogen atom in extractant may be protonated to produce the quaternary amine in acidic condition. From the present work, the maximum distribution ratio of Rh(III) is 16. The D(Rh) values are changeable during preparation of the aqueous solutions because different Rh-Cl-H O complexes are formed in HCl media and show the slow exchange rate between Cl and H
O complexes are formed in HCl media and show the slow exchange rate between Cl and H O. Using the UV spectrum, Rh-chloride solution having the peak of spectrum at 504 nm can be extracted effectively, where RhCl
O. Using the UV spectrum, Rh-chloride solution having the peak of spectrum at 504 nm can be extracted effectively, where RhCl (H
(H O)
O) and RhCl
and RhCl (H
(H O)
O)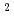
 exist mainly from DFT calculation. Stoichiometry of one-one complex of Rh and NTAamide is obtained from slope analysis, and 85 mM of concentrated Rh ion can be extracted.
exist mainly from DFT calculation. Stoichiometry of one-one complex of Rh and NTAamide is obtained from slope analysis, and 85 mM of concentrated Rh ion can be extracted.
Arai, Yoichi; Watanabe, So; Hasegawa, Kenta; Okamura, Nobuo; Watanabe, Masayuki; Takeda, Keisuke*; Fukumoto, Hiroki*; Ago, Tomohiro*; Hagura, Naoto*; Tsukahara, Takehiko*
Nuclear Instruments and Methods in Physics Research B, 542, p.206 - 213, 2023/09
Times Cited Count:0 Percentile:0.02(Instruments & Instrumentation)Narita, Hirokazu*; Maeda, Motoki*; Tokoro, Chiharu*; Suzuki, Tomoya*; Tanaka, Mikiya*; Shiwaku, Hideaki; Yaita, Tsuyoshi
RSC Advances (Internet), 13(25), p.17001 - 17007, 2023/06
Times Cited Count:1 Percentile:51.1(Chemistry, Multidisciplinary)no abstracts in English
Iwamoto, Toshihiro; Saito, Madoka*; Takahatake, Yoko; Watanabe, So; Watanabe, Masayuki; Naruse, Atsuki*; Tsukahara, Takehiko*
Proceedings of 30th International Conference on Nuclear Engineering (ICONE30) (Internet), 4 Pages, 2023/05
Applicability of temperature swing extraction technology employing monoamides was examined for uranium contaminated waste treatment procedure. Separation experiments on simulated target solution with three kinds of monoamides with different structure showed that Ce(IV) in the solution was selectively recovered by the temperature swing extraction operation. Based on the experiments, an appropriate monoamide for the procedure was selected.
Simonnet, M.; Sittel, T.*; We ling, P.*; Geist, A.*
ling, P.*; Geist, A.*
Energies (Internet), 15(20), p.7724_1 - 7724_10, 2022/10
Times Cited Count:1 Percentile:10.66(Energy & Fuels)Sasaki, Yuji
Bunri Gijutsu, 52(2), p.103 - 107, 2022/03
We develop all-inclusive partitioning method for actinides and fission products in high-level radioactive waste. This process is based on the sequential solvent extraction. In order to recover Cs and Sr for the management by interim storage, crown ether compounds are employed. For the removal of Pd and Mo due to production of a stable vitrified object, methylimino-dioctylacetamide (MIDOA) is taken as an extractant. DGA can extract both actinides and trivalent lanthanides. In order to separate each other, dietylenetriamine-triacetic-diamide (DTBA) for the stripping reagent of MA. For the mutual separation of Am/Cm, DGA and DOODA extraction system is taken into consideration.
Matsutani, Takafumi; Sasaki, Yuji; Katsuta, Shoichi*
Analytical Sciences, 37(11), p.1603 - 1609, 2021/11
Times Cited Count:7 Percentile:48.15(Chemistry, Analytical)We investigated the chemical behavior of lanthanides (Ln) using diglycolamide extractant with multistage extraction. We obtained the breakthrough curves for light and middle Ln. Our study reveals that the metal extraction limit depends on their  values and metal concentrations used in the experiments. From the multistage extractions of 15 aqueous phases and 15 organic phases, three curves (extraction curves, back-extraction curves, and separation curves) were obtained by changing the nitric acid concentration. As an example, under a condition of the separation curve experiment (aqueous phase: 0.5 M HNO
values and metal concentrations used in the experiments. From the multistage extractions of 15 aqueous phases and 15 organic phases, three curves (extraction curves, back-extraction curves, and separation curves) were obtained by changing the nitric acid concentration. As an example, under a condition of the separation curve experiment (aqueous phase: 0.5 M HNO , organic phase: 0.1 M TDDGA (
, organic phase: 0.1 M TDDGA (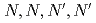 -tetradecyl-diglycolamide) in
-tetradecyl-diglycolamide) in  -dodecane), a recovery of more than 99% of Sm in the organic phase with less than 1% Nd can be obtained.
-dodecane), a recovery of more than 99% of Sm in the organic phase with less than 1% Nd can be obtained.
Kato, Takuma*; Nagaoka, Mika; Guo, H.*; Fujita, Hiroki; Aida, Taku*; Smith, R. L. Jr.*
Environmental Science and Pollution Research, 28(39), p.55725 - 55735, 2021/10
Times Cited Count:0 Percentile:0(Environmental Sciences)In this work, hydrothermal leaching was applied to simulated soils (clay minerals vermiculite, montmorillonite, kaolinite) and actual soils (Terunuma, Japan) to generate organic acids with the objective to develop an additive-free screening method for determination of Sr in soil. Stable strontium (SrCl ) was adsorbed onto soils for study and ten organic acids were evaluated for leaching Sr from simulated soils under hydrothermal conditions (120 to 200
) was adsorbed onto soils for study and ten organic acids were evaluated for leaching Sr from simulated soils under hydrothermal conditions (120 to 200 C) at concentrations up to 0.3 M. For strontium-adsorbed vermiculite (Sr-V), 0.1 M citric acid was found to be effective for leaching Sr at 150
C) at concentrations up to 0.3 M. For strontium-adsorbed vermiculite (Sr-V), 0.1 M citric acid was found to be effective for leaching Sr at 150 C and 1 h treatment time. Based on these results, the formation of organic acids from organic matter in Terunuma soil was studied. Hydrothermal treatment of Terunuma soil produced a maximum amount of organic acids at 200
C and 1 h treatment time. Based on these results, the formation of organic acids from organic matter in Terunuma soil was studied. Hydrothermal treatment of Terunuma soil produced a maximum amount of organic acids at 200 C and 0.5 h reaction time. To confirm the possibility for leaching of Sr from Terunuma soil, strontium-adsorbed Terunuma soil (Sr-S) was studied. For Sr-S, hydrothermal treatment at 200
C and 0.5 h reaction time. To confirm the possibility for leaching of Sr from Terunuma soil, strontium-adsorbed Terunuma soil (Sr-S) was studied. For Sr-S, hydrothermal treatment at 200 C for 0.5 h reaction time allowed 40% of the Sr to be leached at room temperature, thus demonstrating an additive-free method for screening of Sr in soil. The additive-free hydrothermal leaching method avoids calcination of solids in the first step of chemical analysis and has application to both routine monitoring of metals in soils and to emergency situations.
C for 0.5 h reaction time allowed 40% of the Sr to be leached at room temperature, thus demonstrating an additive-free method for screening of Sr in soil. The additive-free hydrothermal leaching method avoids calcination of solids in the first step of chemical analysis and has application to both routine monitoring of metals in soils and to emergency situations.
Simonnet, M.; Kobayashi, Toru; Shimojo, Kojiro; Yokoyama, Keiichi; Yaita, Tsuyoshi
Inorganic Chemistry, 60(17), p.13409 - 13418, 2021/09
Times Cited Count:15 Percentile:87.32(Chemistry, Inorganic & Nuclear)Sasaki, Yuji; Morita, Keisuke; Matsumiya, Masahiko*; Ono, Ryoma*; Shiroishi, Hidenobu*
JOM, 73(4), p.1037 - 1043, 2021/04
Times Cited Count:4 Percentile:34.69(Materials Science, Multidisciplinary)The separation of Dy from Nd is studied from the viewpoint of recycling Dy from Nd magnets. Both metals are lanthanide elements, which means their mutual separation is difficult because of their similar chemical behaviors. All lanthanide elements can be extracted easily by using tetradodecyl-diglycolamide (TDdDGA) extractants, and it has a relatively high separation factor (SF) between Dy and Nd (SF over 10). In the present study, by performing eight extraction steps with the organic phase (0.1M TDdDGA in dodecane), ten steps with an aqueous phase (0.7 M HNO with metals), and six steps with another aqueous phase (0.7 M HNO
with metals), and six steps with another aqueous phase (0.7 M HNO without metals), approximately 99% Dy was recovered into the organic phase with 1% co-extraction of Nd.
without metals), approximately 99% Dy was recovered into the organic phase with 1% co-extraction of Nd.
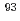 Zr and
Zr and 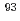 Mo in concrete rubble
Mo in concrete rubbleDo, V. K.; Furuse, Takahiro; Murakami, Erina; Aita, Rena; Ota, Yuki; Sato, Soichi
Journal of Radioanalytical and Nuclear Chemistry, 327(1), p.543 - 553, 2021/01
Times Cited Count:5 Percentile:65.59(Chemistry, Analytical)A new HCl-free chromatographic separation procedure has been developed for sequential separation of Zr and Mo from concrete matrices. Accordingly, 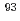 Zr and
Zr and 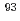 Mo could be sensitively and selectively measured by ICP-MS/MS using ammonia reaction gas. The recoveries of greater than 90% for Zr and Mo from concretes could be achieved. The measurement condition was optimized for complete suppression of interferences from
Mo could be sensitively and selectively measured by ICP-MS/MS using ammonia reaction gas. The recoveries of greater than 90% for Zr and Mo from concretes could be achieved. The measurement condition was optimized for complete suppression of interferences from 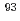 Nb and peak tailing from abundant isotopes of Zr and Mo in concrete matrices. The removal of interferences was verified by measurement of radio-contamination-free concretes used as a sample matrix blank. Method detection limits of 1.7 mBq g
Nb and peak tailing from abundant isotopes of Zr and Mo in concrete matrices. The removal of interferences was verified by measurement of radio-contamination-free concretes used as a sample matrix blank. Method detection limits of 1.7 mBq g and 0.2 Bq g
and 0.2 Bq g were achieved for
were achieved for 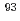 Zr and
Zr and 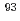 Mo, respectively, in the concrete matrices. The interference removal factor for Nb (equivalent to the decontamination factor in radiochemical separation) was of the order of 10
Mo, respectively, in the concrete matrices. The interference removal factor for Nb (equivalent to the decontamination factor in radiochemical separation) was of the order of 10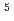 , and the abundance sensitivity was of the order of 10
, and the abundance sensitivity was of the order of 10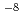 , indicating that the developed method is reliable for verifying the presence of ultralow concentrations of
, indicating that the developed method is reliable for verifying the presence of ultralow concentrations of 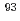 Zr and
Zr and 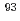 Mo. The present method is suitable for the rapid assessment of
Mo. The present method is suitable for the rapid assessment of 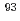 Zr and
Zr and 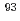 Mo for radioactivity inventory of concrete rubble.
Mo for radioactivity inventory of concrete rubble.
Sasaki, Yuji; Morita, Keisuke; Kitatsuji, Yoshihiro; Ito, Keisuke*; Yoshizuka, Kazuharu*
Solvent Extraction Research and Development, Japan, 28(2), p.121 - 131, 2021/00
Times Cited Count:2 Percentile:14.88(Chemistry, Multidisciplinary)High concentration of Cs is present in high-level radioactive waste. It is well-known that Cs is an alkali element and difficult to extract completely into an organic phase. Crown ether compounds are widely available for Cs extractants; DtBuDB18C6 (di-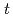 -butyl-dibenzo-18crown6), was used in this study. Organic solvents used for the industrial applications, such as
-butyl-dibenzo-18crown6), was used in this study. Organic solvents used for the industrial applications, such as  -dodecane and 1-octanol, have low solubility concerning the compound; other solvents were employed and tested. In this study, ketone-, ether-, and ester-type solvents showed high solubility for DtBuDB18C6 and DtBuDB18C6, when dissolved in ketones and alcohols, exhibited relatively high Cs distribution ratios (
-dodecane and 1-octanol, have low solubility concerning the compound; other solvents were employed and tested. In this study, ketone-, ether-, and ester-type solvents showed high solubility for DtBuDB18C6 and DtBuDB18C6, when dissolved in ketones and alcohols, exhibited relatively high Cs distribution ratios ( (Cs)), closely to 10.
(Cs)), closely to 10.
Toigawa, Tomohiro; Murayama, Rin*; Kumagai, Yuta; Yamashita, Shinichi*; Suzuki, Hideya; Ban, Yasutoshi; Matsumura, Tatsuro
UTNL-R-0501, p.24 - 25, 2020/12
This report summarizes the results obtained in FY2019 at Electron Linac Facility of University of Tokyo. The radiolysis process of a diglycolamide extractant, which is expected to be used in the separation process of minor actinides (MA), in dodecane and octanol solutions was investigated by pulse radiolysis. As a result, it was suggested that by adding alcohol, the decomposition process of the diglycolamide extractant was different from the decomposition processes in the single solvent of dodecane considered that the decomposition occurred via a radical cation species of the extractant.
Sasaki, Yuji; Nakase, Masahiko*
Petorotekku, 43(11), p.782 - 787, 2020/11
As analog compounds of DGA (diglycolamide), MIDOA(methylimino-diacetamide) and TDGA(thia-diglycolammide) are used for the extractants of platinum group metals. These extractants can be extracted noble metals and oxyanions, which followed by HSAB theory. The high concentration of these metals can be also extracted by these compounds. The research of metal-complex structures gives the information on the ability and role for complex-formation, which will be useful for the development of novel extractants.
 system
systemSasaki, Yuji; Morita, Keisuke; Matsumiya, Masahiko*; Nakase, Masahiko*
Radiochimica Acta, 108(9), p.689 - 699, 2020/09
Times Cited Count:9 Percentile:75.92(Chemistry, Inorganic & Nuclear)The simultaneous separation of Am and Cm from lanthanides is important for atomic energy fields. All lanthanides, Am, and Cm can be extracted by diglycolamide (DGA). In addition, relatively high separation factors between An and Ln were obtained by the extraction system of TODGA, DTPA (diethylenetriamine-pentaacetic acid) and HNO . In this work, DTPA-BA (diethylenetriamine-triacetic-bisamide), which is an improved version of DTPA, was employed for the separation of Ln and An. A relatively high separation factor (approximately 8) for actinides/lanthanides was obtained. Then, the multi-step extraction was performed. Thus, the recoveries of 94.7% for Nd and 4.7% for Am and Cm in organic phase, and 5.3% Nd and 95.3% for Am and Cm in aqueous phase were obtained.
. In this work, DTPA-BA (diethylenetriamine-triacetic-bisamide), which is an improved version of DTPA, was employed for the separation of Ln and An. A relatively high separation factor (approximately 8) for actinides/lanthanides was obtained. Then, the multi-step extraction was performed. Thus, the recoveries of 94.7% for Nd and 4.7% for Am and Cm in organic phase, and 5.3% Nd and 95.3% for Am and Cm in aqueous phase were obtained.
Aono, Ryuji; Mitsukai, Akina; Haraga, Tomoko; Ishimori, Kenichiro; Kameo, Yutaka
JAEA-Data/Code 2020-006, 70 Pages, 2020/08
Radioactive wastes which generated from research and testing reactors in Japan Atomic Energy Agency are planning to be buried at the near surface disposal field. Therefore, it is required to establish the method to evaluate the radioactivity concentrations of radioactive wastes by the time it starts disposal. In order to contribute to this work, we collected and analyzed the samples generated from JPDR and JRR-4. In this report, we summarized the radioactivity concentrations of 19 radionuclides ( H,
H,  C,
C, 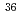 Cl,
Cl, 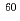 Co,
Co, 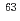 Ni,
Ni,  Sr,
Sr, 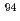 Nb,
Nb,  Tc,
Tc, 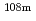 Ag,
Ag, 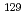 I,
I,  Cs,
Cs, 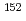 Eu,
Eu, 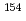 Eu,
Eu, 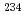 U,
U, 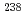 U,
U, 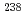 Pu,
Pu, 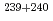 Pu,
Pu, 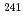 Am,
Am, 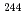 Cm) which were obtained from radiochemical analysis of those samples.
Cm) which were obtained from radiochemical analysis of those samples.
Sato, Junya; Shiota, Kenji*; Takaoka, Masaki*
Journal of Hazardous Materials, 385, p.121109_1 - 121109_9, 2020/03
Times Cited Count:9 Percentile:43.42(Engineering, Environmental)Lead is a hazardous heavy metal that can be stabilized by incorporation into the matrix of aluminosilicate bearing phases as they solidify. The actual mechanism by which lead is stabilized, however, continues to be unclear because the individual mechanisms of Pb incorporation into crystalline and amorphous aluminosilicate phases have not yet been studied separately. A detailed investigation of the incorporation of Pb into the amorphous phase of aluminosilicate solids was therefore performed. Amorphous aluminosilicate solids were synthesized with 0.7, 1.5, and 3.7 wt% of Pb from aluminosilicate gel produced from chemical reagents. Based on Raman spectroscopy, the Si-O stretching vibration bond shifted to lower wavenumbers with increasing Pb concentration. This shift suggested that covalent bonding between Pb and O in the matrix of the aluminosilicate solids increased. In addition, sequential extraction revealed that most of the Pb (75-90%) in the aluminosilicate solids was in a poorly soluble form (i.e. reducible, oxidizable, and residual fractions). These findings indicate that most of Pb is bonded covalently to the amorphous phase in aluminosilicate solids.
Simonnet, M.; Suzuki, Shinichi; Miyazaki, Yuji*; Kobayashi, Toru; Yokoyama, Keiichi; Yaita, Tsuyoshi
Solvent Extraction and Ion Exchange, 38(4), p.430 - 440, 2020/00
Times Cited Count:18 Percentile:69.88(Chemistry, Multidisciplinary)